
I deal with a complex and recent subject that is of great importance in monitoring patients afflicted with ARDS: the Patient Self-Inflicted Lung Injury (P-SILI).
The objectives of this piece are divided into two parts:
- To define the P-SILI.
- Bedside monitoring of P-SILI (which we will discuss in the second part of this report)
DEFINING P-SILI
Over the past 20 years, several important aspects concerning ARDS patients have been emphasized. This includes: invasive and non-invasive ventilatory treatments, the timing of intubation, permissive CO2, prone positioning, awake proning, and ventilator-induced lung injury (VILI) from its main mechanisms such as volutrauma, barotrauma, atelectrauma, biotrauma, to the monitoring of the driving pressure and transpulmonary pressure as a guide for conducting protective ventilation.
P-SILI stands for patient-inflicted acute lung injury, an acronym that has recently stood out in the nursing community, was rarely used until now, mainly due to the pandemic. Ever since 2020, it has been mentioned more often.
The P-SILI physiological concept has had roots and experimental conducted over the past 30 years. Its acronym, however was coined more recently by the Canadian group led by Laurent Brochard (3).
The P-SILI expands the concept of VILI in a more global vision of the induced lung injury as the VILI can conceptually occur only undergoing mechanical ventilation, while the P-SILI can occur in severe ARDS cases, especially during spontaneous breathing.
In simple words, we can define P-SILI as lung damage caused by respiratory muscle activity in patients with severe ARDS, spontaneous breathing, and undergoing invasive or noninvasive mechanical ventilation.
Pathophysiology of P-SILI
From a physiological point of view, spontaneous breathing during mechanical ventilation provides various benefits such as:
- Maintenance of end-expiratory lung volume;
- Predominant back ventilation;
- Better alveolar gas exchange;
- Prevention of diaphragmatic dysfunction.
Spontaneous breathing during mechanical ventilation, consequently, has been encouraged for quite some time to be maintained during mechanical ventilation. Recent studies, however, have been debating the negative effects of spontaneous activity, especially in the more severe forms of ARDS (3) (4) (5) (6) (7) (8) (9) (10), both in patients with spontaneous breathing and patients undergoing mechanical invasive or noninvasive ventilation (11). It is necessary to point out that P-SILI is not caused by spontaneous breathing, but rather by an intense inspiratory effort known as a high, or oscillation of the pleural pressure (∆Ppl), which can occur in these types of patients. The pathological respiratory drive is caused by the response that the respiratory center puts in place as the result of stimuli coming from the structures above the Pontine (anxiety, pain, discomfort); inflammatory inputs caused by inflammatory mediators (histamine, bradykinin, and prostaglandins) constantly activated by pulmonary edema; chemical inputs caused by PaO2 reduction, increased CO2 and acidosis and finally the mechanical inputs caused by atelectasis/derecruitment. (12)
The excessive inspiratory effort could induce those mechanisms, which are responsible for the lung damage, the increase in tidal volume, as a result of pulmonary over-expansion, the pendelluft effect, and the increase of pulmonary edema.
To understand the mechanism that causes P-SILI, one must start with a very simple assumption: the phenomenon that is primarily responsible for causing or worsening lung injury during invasive and non-invasive mechanical ventilation is the over-distention of adequately ventilated areas.
This phenomenon usually occurs when the tidal volume or the set inspiratory assistance are excessive for a pathological “small” lung that is considered a “baby lung” (13).
Overstretch conditions can also occur due to elevated transpulmonary pressure during inspiration. This is often responsible for large VT, which results in increased stress and pulmonary strain. Even patient-ventilator asynchronies, caused by high inspiratory effort, such as the double triggering and the reverse triggering, can often lead to a false double triggering, causing over-distention phenomena.
As shown in image 1, despite the setting of protective ventilation, the patient initiates two inflations with a single muscular effort and consequent over-distention of the lung.
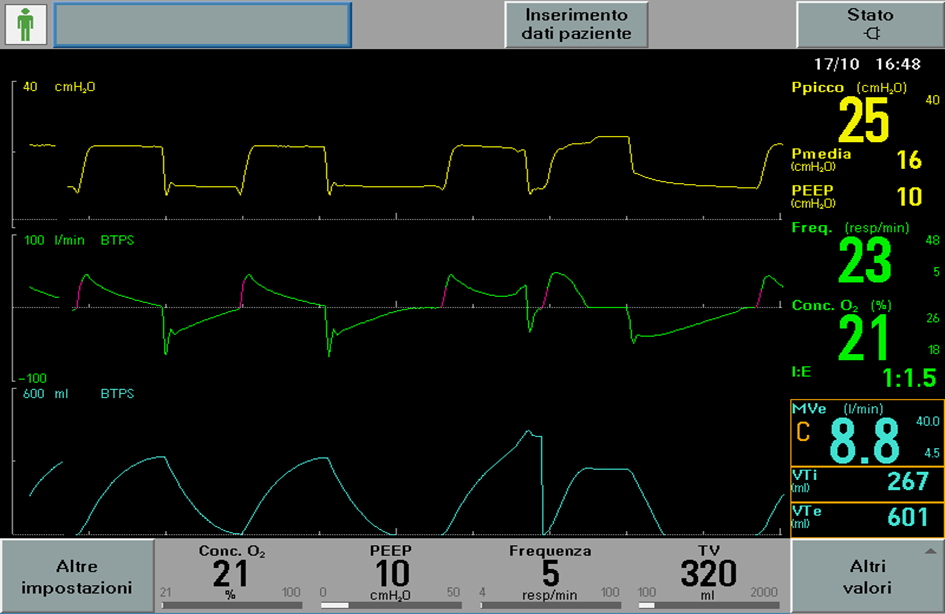
In this patient category, lung damage could occur even if it’s not related to an increase in inspired volumes as it so happens during Pendelluft effect.
ARDS patients in the supine or semi-recumbent position subject their dorsal lung regions (defined as “dependent”) to an increase in gravitational pressure, which are small or not ventilated at all, while the ventral areas (defined as “non-dependant”) are adequately ventilated.
The distribution of the tidal volume within the lungs is generally more homogeneous during special spontaneous breathing than during controlled mechanical ventilation. The excessive effort, however, can lead to heterogeneity of the ventilation with a greater proportion of tidal volumes reaching the dependent regions. This phenomenon can occur both in spontaneous breathing and during mechanical ventilation when an uncontrolled inspiratory effort occurs, known as the “Pendelluft” effect. Even in the absence of high title volumes, a regional injury may occur due to an increase in local stress in the dependent atelectasis lung regions. The local forces extruded on the lung by the respiratory muscles could generate the movement of gas from non-dependent zones to dependent zones with consequent stress and damage to the dependent zones. This takes place because the decrease in pleural pressure generated by diaphragmatic contraction is greater than the dependent lung regions (image 2), which subtracts air from non-dependent lung regions before the ventilator flows reach the alveoli (an occult phenomenon of Pendelluft) (5).
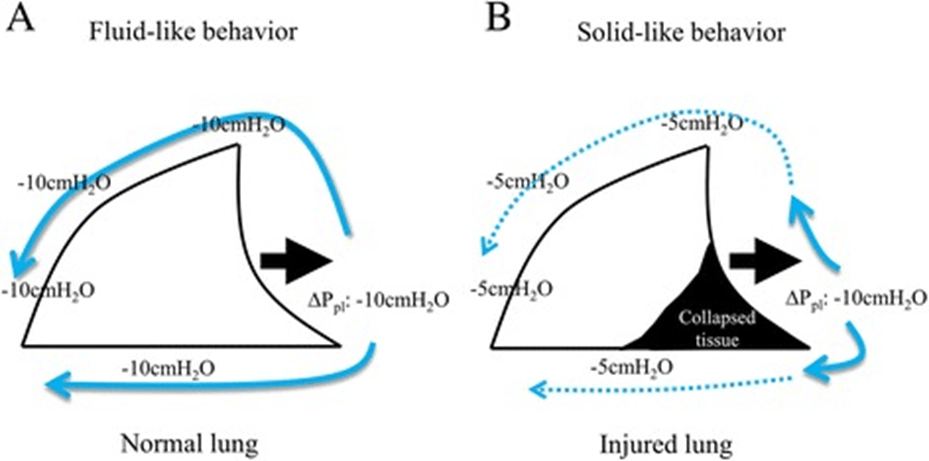
During inspiratory efforts, intrathoracic pressure is reduced and the volume of intrathoracic blood increases. During these efforts, the intravascular pressure measured in the pulmonary intrathoracic vessels decreases, but to a lesser extent than the fall in pleural pressure, resulting in increased trans-vascular pressures that can lead to the genesis of a pulmonary edema or its worsening (image 3).
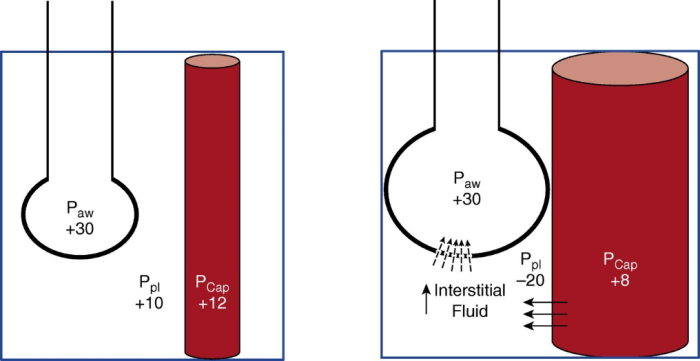
As shown in image 3, during a mechanical breath with a passive patient (figure 3 left) trans-pulmonary pressure (PL), which stretches the lung is +20 cmH2O (PL= Palv – Ppl= 30 – 10); the blood vessels and lungs are compressed by the breath positive pressure, while the trans-vascular pressure (Pcap – Ppl) it’s low (ex 12 – 10 = 2 cmH2O) therefore there are less favourable conditions for the migration of fluids from the vessels to the interstitial. When strong spontaneous inspiratory effort (figure 3 right) is added, the PL (30 + 20 = +50 cmH2O) is increased, thus increasing the tidal volume and causing lung injury. Furthermore, a very negative (−20 cmH2O) Ppl distends blood vessels and lungs, increasing perfusion, but creating a greater trans-vascular pressure (ex 8 – (- 20) = +28 cmH2O), thus favouring the movement of liquids towards the interstitial (7). In the presence of lesions, as it occurs in ARDS, the increased permeability of the alveolar-capillary barrier can only favour the development of alveolar edema. Edema can reduce lung compliance and increase ventilation heterogeneity, thereby contributing to additional aggravation.
In the next Post, we will deal with the understanding of P-SILI at the patient’s bedside.
Stefano Parise and Enrico Bulleri
English version edited by Giulia Azzini
Bibliography
- Dreyfuss D, Soler P, Basset G, Saumon G. High inflation pressure pulmonary edema. Respective effects of high airway pressure, high tidal volume, and positive end-expiratory pressure. Am Rev Respir Dis. 1988;137(5):1159‐1164
- Mascheroni D, Kolobow T, Fumagalli R, Moretti MP, Chen V, Buckhold D. Acute respiratory failure following pharmacologically induced hyperventilation: an experimental animal study. Intensive Care Med. 1988;15(1):8‐14
- Brochard L, Slutsky A, Pesenti A. Mechanical Ventilation to Minimize Progression of Lung Injury in Acute Respiratory Failure. Am J Respir Crit Care Med. 2016;195(4):438‐442
- Bertoni M, Telias I, Urner M, et al. A novel non-invasive method to detect excessively high respiratory effort and dynamic transpulmonary driving pressure during mechanical ventilation. Crit Care. 2019;23(1):346
- Yoshida T, Torsani V, Gomes S, et al. Spontaneous effort causes occult pendelluft during mechanical ventilation. Am J Respir Crit Care Med. 2013;188(12):1420‐1427
- Yoshida T, Uchiyama A, Fujino Y. The role of spontaneous effort during mechanical ventilation: normal lung versus injured lung. J Intensive Care. 2015 Jun 17; 3:18.
- Yoshida T, Fujino Y, Amato MB, Kavanagh BP. Fifty Years of Research in ARDS. Spontaneous Breathing during Mechanical Ventilation. Risks, Mechanisms, and Management. Am J Respir Crit Care Med. 2016;195(8):985‐992
- Yoshida T, Amato MBP, Kavanagh BP, Fujino Y. Impact of spontaneous breathing during mechanical ventilation in acute respiratory distress syndrome. Curr Opin Crit Care. 2019 Apr;25(2):192-198
- Bellani G, Grasselli G, Teggia-Droghi M, et al. Do spontaneous and mechanical breathing have similar effects on average transpulmonary and alveolar pressure? A clinical crossover study. Crit Care. 2016;20(1):142
- Bellani G, Grassi A, Sosio S, Gatti S, Kavanagh BP, Pesenti A, et al. Driving Pressure Is Associated with Outcome during Assisted Ventilation in Acute Respiratory Distress Syndrome. Anesthesiology. 2019;131(3):594-604
- Bellani G, Grassi A, Sosio S, Foti G. Plateau and driving pressure in the presence of spontaneous breathing. Intensive Care Med. 2019;45(1):97‐98
- Spinelli E, Mauri T, Beitler JR, Pesenti A, Brodie D. Respiratory drive in the acute respiratory distress syndrome: pathophysiology, monitoring, and therapeutic interventions. Intensive Care Med. 2020;46(4):606‐618
- Gattinoni L, Pesenti A (2005) The concept of “baby lung”. Intensive Care Med 31:776–784