Morgenstern, J. INTERACT3: Management of intracranial hemorrhage, First10EM, November 27, 2023. Available at:
https://doi.org/10.51684/FIRS.132873
Evidentiary amnesia is a term I use to describe the phenomenon I frequently encounter in which evidence seems to be forgotten over time. When I hear people lecture about the management of intracranial hemorrhage, and even when I invite incredibly smart people to write about neurologic emergencies, blood pressure management is always recommended. But why? We have two large RCTs that look at this issue. The INTERACT 2 trial was a multi-center, randomized, partially blinded trial comparing intensive blood pressure control (target of a systolic pressure <140 within 1 hour) to guideline recommended care (to a target systolic <180) in 2794 adult patients with intracerebral hemorrhage within the last 6 hours, and there was no difference in primary outcome of death or disability at 90 days (52.0% vs 55.6%, p=0.06). (Anderson 2013) Obviously this was close to statistically significant, and does not exclude the possibility of benefit, but we have another large RCT to consider as well. The ATTACH-2 trial was another randomized, multi-center, open-label trial that compared intensive blood pressure management (target systolic 110-139) to standard BP management (target 140-179) in 1000 patients with acute intracranial hemorrhage, and there was absolutely no benefit this time around (death or disability was 38.7% with intensive care vs 37.7% with standard care.) (Qureshi 2016) Therefore, to date there is no evidence that blood pressure control helps our patients. For the vast majority of these patients, the only blood pressure management I provide is adequate analgesia.
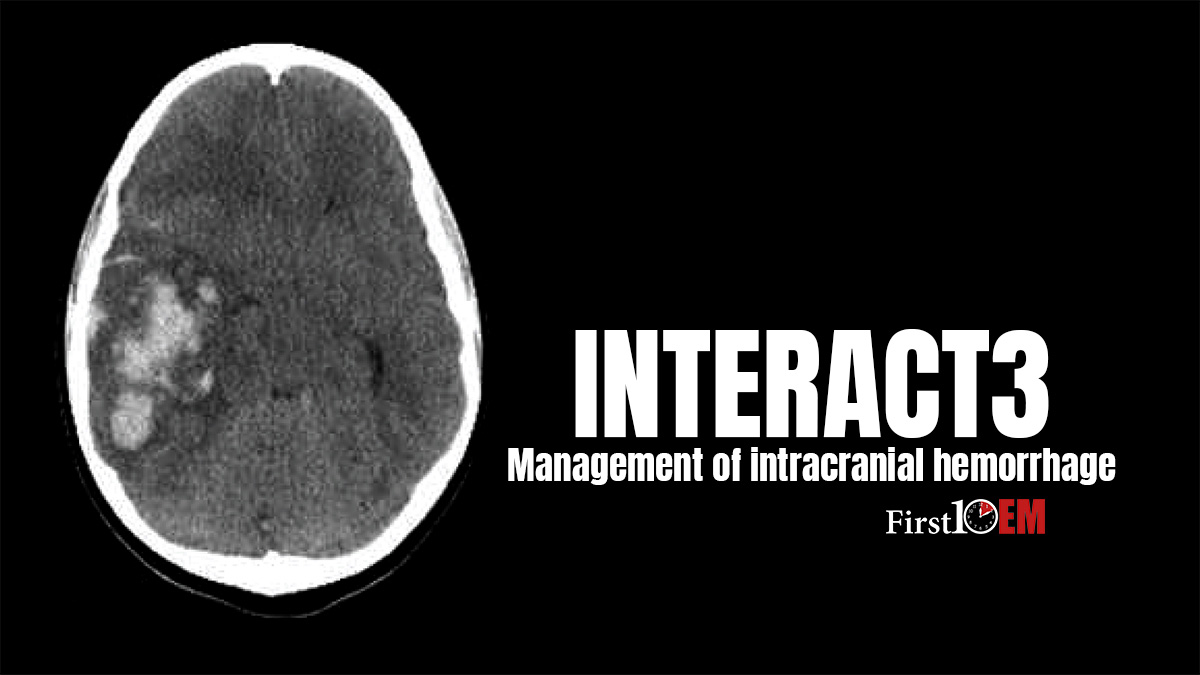
However, I have recently heard rumblings that intensive blood pressure management is now proven, based on the results of the INTERACT3 trial. So what did INTERACT 3 actually demonstrate?
The paper
INTERACT3: Ma L, Hu X, Song L, Chen X, et al; INTERACT3 Investigators. The third Intensive Care Bundle with Blood Pressure Reduction in Acute Cerebral Haemorrhage Trial (INTERACT3): an international, stepped wedge cluster randomised controlled trial. Lancet. 2023 Jul 1;402(10395):27-40. doi: 10.1016/S0140-6736(23)00806-1. PMID: 37245517 NCT03209258
The Methods
INTERACT3 was a pragmatic international, multicentre, stepped-wedge cluster RCT enrolling patients in Brazil, China, India, Mexico, Nigeria, Pakistan, Peru, Sri Lanka, Viet Nam, and Chile.
Participants
Hospital sites with no existing protocol for managing abnormal physiologic variables in head bleed patients were identified through neurosurgery professional networks. The target was primarily low and middle income countries. Once identified, hospitals had to include consecutive intracranial hemorrhage patients who presented within 6 hours of symptom onset, except those with a bleed secondary to a structural abnormality or reperfusion therapy, or patients unlikely to adhere to treatment or follow-up.
Intervention
A bundle of care that included early intensive blood pressure management (target systolic blood pressure less than 140 mm Hg within 1 hour), intensive glucose control (target 6.1-7.8 mmol/L in patient without diabetes and 7.8-10.0 for those with), treatment of pyrexia (with a target of a temperature less than 37.5 Celsius), and reversal of warfarin (with either FFP or PCC and a target INR of 1.5) within 1.5 hours. All targets were maintained for 7 days.
Comparison
Usual care.
Outcome
The primary outcome was functional recovery at 6 months based on the modified Rankin scale, defining scores of 0 and 1 as favourable.
The Results
They identified 144 eligible hospitals, of which 122 ultimately contributed patients. 10,857 patients were screened and 7,036 were enrolled. (Their calculated target sample size was 8,360, and, unless I missed it, they don’t really explain why they stopped before this target, but I believe COVID and slow recruitment were culprits.)
The mean age of patients was 62 years. 36% were female. Almost all (90%) were Chinese. Presenting GCS was a median of 12, and a median NIHSS score of 13. The median hematoma size was 15 mL.
Using an ordinal analysis, the patients in the intervention group had better functional outcomes on the modified Rankin score (OR 0.86, 95% CI 0.76-0.97, p=0.015).
The bundled care groups also had a lower odds of death (OR 0.77, 95% CI 0.63-0.95), although that difference was not statistically significant after their planned adjustments.
There was no statistical difference in the more standard dichotomous outcomes, using a mRs 0-2 as the good outcome (OR 0.89, 95%CI 0.78-1.02).
My thoughts
INTERACT3 is an interesting trial, but I don’t think it should result in any significant change in clinical practice.
Although hospitals were randomized as to when they would start this new intervention, there are no real control groups here, and the trial is unblinded. This trial design is really not different from a standard before/after observational study, and all the problems with before/after research apply. (They randomized when to start the before/after intervention, but each hospital was just a normal before/after observational trial.) The biggest issue is likely the Hawthorne effect, in that people know their actions are being monitored, and the new protocol will definitely bring far more nursing attention to the patient. Better outcomes might have nothing to do with the protocol itself, but simply increased attentiveness to the patients. In that way, the trial clearly demonstrates that there is room to improve care of patients with intracranial hemorrhage in these LMIC hospitals with no existing management protocols, but it doesn’t tell us what specific interventions actually help, and the benefit might be entirely from increased nursing attention.
This trial is not equivalent to a RCT. It is essentially observational data, although very well done observational data, and as such, its results shouldn’t overrule existing RCT data. Thus, for a topic like blood pressure targets, where we have existing large and high quality RCTs demonstrating no benefit, this trial does not provide any impetus for practice change. (Moullaali 2022)
The biggest problem with any study looking at a bundle of care is that we have no idea which, if any, of the multiple interventions actually help. In fact, it is possible that some interventions were actually harmful, but that harm was just outweighed by other interventions.
Based on the data they provide, we can try to guess what interventions might have helped. It seems pretty unlikely blood pressure management helped, given that the rate of antihypertensive therapy was similar in both groups (70% vs 78%), and the mean systolic blood pressures were almost identical (6 mm Hg difference at 1 hour and 3 mm HG difference at 24 hours). When combined with the fact that we have multiple proper RCTs (INTERACT2 and ATTACH2) that show no benefit from blood pressure management in these patients, we can be reasonably certain that the blood pressure component of this bundle had nothing to do with the outcomes. (Anderson 2013; Qureshi 2016; Moullaali 2022)
It also seems unlikely that intensive glucose control made a big difference, because even though more patients were within the target range in the treatment groups (13% vs 7%), the mean glucose difference between the two groups was only 0.5 mmol/L. Similar to blood pressure control, we have proper RCTs looking at intensive glucose control (although in somewhat different populations), and the results have not been great.
The number of patients that received anti-pyrexia treatment was also very similar (9.8% vs 7.4%), and the percentage of patients hitting the target of <37.5 Celsius was basically the same (87% vs 84%). Once again, there is no RCT evidence that temperature control helps in any patient population, so it seems unlikely that temperature control is the key intervention.

There was a slight benefit in terms of the number of patients who achieved an INR of less than 1.5 (48% vs 41%), which you could imagine might help patients with head bleeds, although the data on anticoagulation reversal actually hasn’t been that promising in the past.
Looking at all these numbers, I think the best explanation for the improved primary outcome is probably bias in this before/after observational data, given that none of the individual interventions seem to provide enough of an effect to explain the improved mortality or functional outcomes described.
There are a number of other issues that need to be considered when analyzing this trial. Selection bias is a concern, as physicians were allowed to exclude anyone they thought might not adhere to the study’s follow-up protocols. (I am also not entirely sure of the rationale for excluding patients with structural causes for their bleeds.) External validity will be a concern, as they specifically targeted low and middle income countries, and one might imagine the available nursing resources might not match what we are used to in North America, which would be particularly important if the entire difference between the groups was the result of increased nursing care. I have discussed problems with the modified Rankin score before, but I think it is important to emphasize that this is a very subjective scale that was measured almost entirely by phone interviews with a caregiver in this unblinded trial, making these results very unreliable.
Bottom line
Although this is a cluster RCT, the strength of evidence is closer to an observational before/after trial. My guess is that the improved outcomes have nothing to do with the individual components of their bundle of care, but instead the added nursing attention such a bundle entails, as well as the bias inherent to a before/after trial design.
With regards to blood pressure management, I think we are left where we were before: the bulk of the evidence suggests no benefit. Blood pressure control is not a priority in these patients. I start with analgesia, which works for the vast majority of patients. There is no evidence of harm, so I start antihypertensives if and when they are requested by my neurosurgery colleagues, but am cautious in my titration to ensure no harm.
Other FOAMed
References
Anderson CS, Heeley E, Huang Y. Rapid blood-pressure lowering in patients with acute intracerebral hemorrhage. The New England journal of medicine. 368(25):2355-65. 2013. PMID: 23713578
INTERACT3: Ma L, Hu X, Song L, Chen X, et al; INTERACT3 Investigators. The third Intensive Care Bundle with Blood Pressure Reduction in Acute Cerebral Haemorrhage Trial (INTERACT3): an international, stepped wedge cluster randomised controlled trial. Lancet. 2023 Jul 1;402(10395):27-40. doi: 10.1016/S0140-6736(23)00806-1. PMID: 37245517
Moullaali TJ, Wang X, Sandset EC, Woodhouse LJ, Law ZK, Arima H, Butcher KS, Chalmers J, Delcourt C, Edwards L, Gupta S, Jiang W, Koch S, Potter J, Qureshi AI, Robinson TG, Al-Shahi Salman R, Saver JL, Sprigg N, Wardlaw JM, Anderson CS, Bath PM; Blood Pressure in Acute Stroke (BASC) Investigators. Early lowering of blood pressure after acute intracerebral haemorrhage: a systematic review and meta-analysis of individual patient data. J Neurol Neurosurg Psychiatry. 2022 Jan;93(1):6-13. doi: 10.1136/jnnp-2021-327195. Epub 2021 Nov 3. PMID: 34732465
Qureshi AI, Palesch YY, Barsan WG. Intensive Blood-Pressure Lowering in Patients with Acute Cerebral Hemorrhage. The New England journal of medicine. 2016. PMID: 27276234





2 thoughts on “INTERACT3: Management of intracranial hemorrhage”
Justin,
I am not sure about there being no harm in starting anti-hypertensives. Where I work, a hypertensive hemorrhagic stroke will be nursed in a resuscitation cubicle, the neurosurgeons will often demand an arterial line, and frequent IV anti-hypertensives will be given. All this consumes resources, something that is hard to justify given the lack of evidence for a benefit from intensive BP control.
I think you are entirely correct. I may have softened my words too much, given the strong opinions in the community for aggressive BP control. Wasn’t looking to start too much controversy, but I don’t do it unless specifically requested by the person assuming responsibility for the patient. How much I should fight over this, given equivalent outcomes in the trial, is the question. My answer thus far has been not much, but I hear your concerns.